In this video, we’ll go over how to prepare and administer TEPEZZA.
First things first. TEPEZZA is supplied as a lyophilized powder for reconstitution.
Each carton contains a single-dose vial with 500 mg of teprotumumab antibody.
Before reconstitution, store TEPEZZA inside the box, protect from light, and refrigerate between 36°F and 46°F. Do not freeze. Okay! Let’s get started. TEPEZZA is given once every 3 weeks for a total of 8 infusions.
It is dosed according to the patient’s actual body weight. Start with 10 mg/kg for the first infusion, followed by 20 mg/kg for infusions 2 to 8. For a helpful dosing calculator, go to TEPEZZAhcp.com.
Let’s gather our supplies. We’ll need the TEPEZZA vial or vials, a sterile syringe and needle, Sterile Water for Injection, USP, an intravenous infusion bag containing 0.9% Sodium Chloride Solution, USP, an infusion administration set, and any routine infusion supplies.
An in-line filter with a 0.2 µm pore size is optional. Importantly, no special tubing is required with TEPEZZA. Remember to check the seal and expiration date on each TEPEZZA vial before use. Now, we’re ready to reconstitute the lyophilized powder. As always, remember to use aseptic technique.
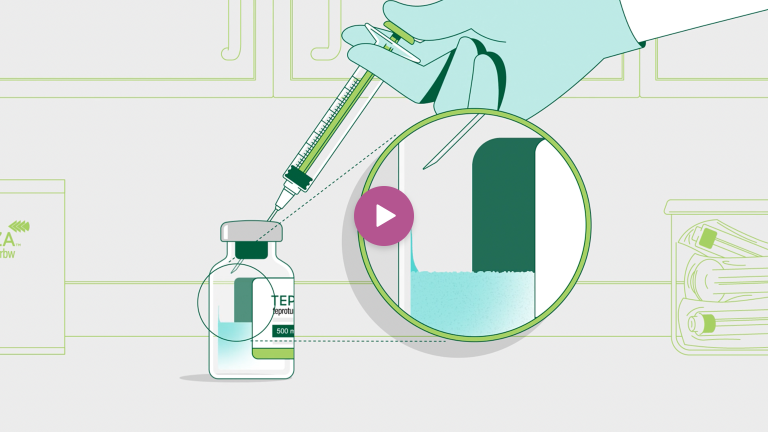
Reconstitute each TEPEZZA vial with 10 mL of Sterile Water for Injection.
Make sure the stream of diluent is not directed onto the cake of lyophilized powder.
When finished, gently swirl the solution by rotating the vial until the powder is dissolved.
Do not shake.
Now, you will have a volume of 10.5 mL at a concentration of 47.6 mg of TEPEZZA per mL.
Before moving on, visually inspect the solution. It should be colorless or slightly brown, clear to opalescent, and free of particulate matter. If not, discard. Next, we’ll further dilute the solution. Select an intravenous infusion bag containing 0.9% Sodium Chloride Solution, USP. If the dose is <1800 mg, use a 100-mL bag. If the dose is ≥1800 mg, use a 250-mL bag. The volume in the infusion bag should remain constant.
Use a sterile syringe and needle to remove the volume of saline equal to the amount of the reconstituted solution to be placed into the bag. Discard the saline withdrawn.
Now, withdraw the required volume from the TEPEZZA vial or vials based on the dose, and transfer into the infusion bag. Mix the diluted solution by gentle inversion. Do not shake.
Discard vials and all unused contents. Keep in mind that the combined storage time of reconstituted solution in the vial and diluted solution in the infusion bag is a total of 4 hours at room temperature or up to 48 hours in refrigeration.
If not administered immediately, protect from light. The TEPEZZA solution is now ready for infusion. If refrigerated, allow the diluted solution to reach room temperature prior to infusion. Infuse the diluted solution over 90 minutes for Infusions 1 and 2. Reduce to 60 minutes for Infusions 3 to 8. If not well tolerated, the minimum infusion time should remain at 90 minutes.
Do not administer as an intravenous push or bolus and do not infuse concomitantly with other agents. During the infusion, follow your institution’s protocol to monitor for infusion reactions. If an infusion reaction occurs, interrupt or slow the rate of infusion and use appropriate medical management.
For subsequent infusions, consider premedicating with an antihistamine, antipyretic, and/or corticosteroid, and consider slowing the rate of infusion.
When the infusion is complete, discard items.
For more information, see the Infusion Guide available at TEPEZZAhcp.com. To contact Horizon for medical information, or to report adverse events and product complaints, please call 1-866-479-6742.
For additional information on TEPEZZA, please see the Full Prescribing Information at TEPEZZAhcp.com.